Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 (M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd
(M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd using cadmium chloride
using cadmium chlorideHayashi, Hirokazu; Shibata, Hiroki; Sato, Takumi; Otobe, Haruyoshi
Journal of Radioanalytical and Nuclear Chemistry, 332(2), p.503 - 510, 2023/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)The formation of MPd (M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu
(M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu -type GdPd
-type GdPd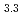 (
( = 0.4081
= 0.4081  0.0001 nm) and NpPd
0.0001 nm) and NpPd (
( = 0.4081
= 0.4081  0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi
0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi -type NpPd
-type NpPd . Chlorination of the MPd
. Chlorination of the MPd (M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl
(M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl formation.
formation.
Ito, Tatsuya; Osugi, Haruka*; Osawa, Naoki*; Takahashi, Tadayuki*; Kim, S.-Y.*; Nagaishi, Ryuji
Analytical Sciences, 38(1), p.91 - 97, 2022/01
Times Cited Count:2 Percentile:15.48(Chemistry, Analytical)A novel ionic liquid (IL) functionalized with thiodiglycol amic acid containing a soft S donor was synthesized for the effective and efficient extraction of platinum group metals (Ru, Rh, and Pd) from aqueous nitric acid solutions, such as high-level radioactive liquid waste (HLLW). The IL allowed a rapid extraction of Pd(II) with an extraction ratio of approximately 100%. The extractions of Ru(III) and Rh(III) by the IL were slower than that of Pd(II), but the rates were accelerated by temperature elevation. The extractions of Ru(III) and Rh(III) at 50 C reached equilibrium within 4 and 8 h, respectively, with the extraction ratios of over 90% without assisting agents or other methods for the extraction system. Furthermore, the IL could extract more than 90% of Ru(III), Rh(III), and Pd(II) from the simulated HLLW within 2 h at 50
C reached equilibrium within 4 and 8 h, respectively, with the extraction ratios of over 90% without assisting agents or other methods for the extraction system. Furthermore, the IL could extract more than 90% of Ru(III), Rh(III), and Pd(II) from the simulated HLLW within 2 h at 50 C.
C.
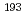 Ir M
Ir M ssbauer spectroscopic parameters of Vaska's complexes and their oxidative adducts
ssbauer spectroscopic parameters of Vaska's complexes and their oxidative adductsKaneko, Masashi; Nakashima, Satoru*
Inorganic Chemistry, 60(17), p.12740 - 12752, 2021/09
Times Cited Count:3 Percentile:32.49(Chemistry, Inorganic & Nuclear)In the present study, density functional theory (DFT) calculation was applied to Vaska's complexes of formula 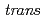 -[IrCl(CO)(PPh
-[IrCl(CO)(PPh )
) ], and their oxidative adducts with small molecules (YZ) including H
], and their oxidative adducts with small molecules (YZ) including H , i.e.,
, i.e., 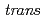 -[IrCl(YZ)(CO))(PPh
-[IrCl(YZ)(CO))(PPh )
) ], to successfully correlate the electronic states of the complexes with the corresponding
], to successfully correlate the electronic states of the complexes with the corresponding 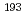 Ir M
Ir M ssbauer spectroscopic parameters. After confirming the reproducibility of the DFT methods for elucidating the equilibrium structures and
ssbauer spectroscopic parameters. After confirming the reproducibility of the DFT methods for elucidating the equilibrium structures and 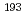 Ir M
Ir M ssbauer isomer shifts of the octahedral Ir complexes, the isomer shifts and quadrupole splitting values of Vaska's complexes and their oxidative adducts were calculated. A bond critical point analysis revealed that the tendency in the isomer shifts was correlated with the strength of the covalent interaction in the coordination bonds. In an electric field gradient (EFG) analysis of the oxidative adducts, the sign of the principal axis was found to be positive for the complex with YZ = Cl
ssbauer isomer shifts of the octahedral Ir complexes, the isomer shifts and quadrupole splitting values of Vaska's complexes and their oxidative adducts were calculated. A bond critical point analysis revealed that the tendency in the isomer shifts was correlated with the strength of the covalent interaction in the coordination bonds. In an electric field gradient (EFG) analysis of the oxidative adducts, the sign of the principal axis was found to be positive for the complex with YZ = Cl and negative for the complex with YZ = H
and negative for the complex with YZ = H . This reversal of the sign of the EFG principal axis was caused by the difference in the electron density distribution for the coordination bonds between Ir and YZ, according to a density of states analysis.
. This reversal of the sign of the EFG principal axis was caused by the difference in the electron density distribution for the coordination bonds between Ir and YZ, according to a density of states analysis.
Mishima, Ria; Inaba, Yusuke*; Tachioka, Sotaro*; Harigai, Miki*; Watanabe, Shinta*; Onoe, Jun*; Nakase, Masahiko*; Matsumura, Tatsuro; Takeshita, Kenji*
Chemistry Letters, 49(1), p.83 - 86, 2020/01
Times Cited Count:4 Percentile:21.18(Chemistry, Multidisciplinary)Separation of platinum group metals (PGMs) from high-level liquid waste generated from the reprocessing of spent nuclear fuels is important to produce good quality vitrified glass for final disposal. A new sorbent, Aluminum hexacyanoferrate (AlHCF), was synthesized and the general sorption behavior of PGMs from concentrated nitric acid was examined. Nitric acid caused substantial elution of AlHCF but the sorption of Pd stabilized the structure. Consequently, Rh was sorbed in the presence of Pd, whereas single Rh sorption caused complete dissolution of AlHCF. Relation between sorbed mount of Pd vs eluted Al and Fe revealed that the elution ratio of Al and Fe was not the same as molar ratio of synthesized AlHCF, indicating the re-sorption of Fe resulted in formation of new structure. The sorption mechanism of PGMs by this new sorbent, AlHCF, not only the simple ion exchange, but also oxidation reduction reaction as well as kinetics play important rule. Understanding the general sorption and dissolution behavior will help improve the sorption performance of PGMs by AlHCF.
Saeki, Morihisa*; Asai, Shiho; Oba, Hironori*
Bunseki, 2018(4), p.138 - 143, 2018/04
Platinum group metal (PGM) has attracted much attention in light of increasing demands in the industrial sector. A wide variety of techniques specialized for PGM separation, such as, solvent extraction, solid phase extraction, and molten salt electrolysis have been developed so far. Among such techniques, a newly developed separation technique based on laser-induced particulate formation can be a promising alternative to conventional ones. It enables non-contact and highly-selective separation with a simple operation. In this review, the research history and the basic mechanism of laser-induced particulate formation were outlined. Several applications were also mentioned, focusing on our latest research progress which achieved a world first quantitation of radioactive palladium in a spent nuclear fuel sample.
Onishi, Takashi; Sekioka, Ken*; Suto, Mitsuo*; Tanaka, Kosuke; Koyama, Shinichi; Inaba, Yusuke*; Takahashi, Hideharu*; Harigai, Miki*; Takeshita, Kenji*
Energy Procedia, 131, p.151 - 156, 2017/12
Times Cited Count:11 Percentile:98.3(Energy & Fuels)no abstracts in English
Okamoto, Yoshihiro; Nagai, Takayuki; Shiwaku, Hideaki; Inose, Takehiko*; Sato, Seiichi*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 16(4), p.180 - 190, 2017/12
X-ray absorption fine structure (XAFS) and imaging XAFS analyses were performed to elucidate chemical state of rhodium in the simulated waste glass. The chemical forms of Rh in the glass were evaluated to be 84% RhO and 16% metal/alloy as the result of linear combination analysis of EXAFS data. According to the imaging XAFS analysis, the chemical form of Rh which was located together with Ru was mainly oxide (RhO
and 16% metal/alloy as the result of linear combination analysis of EXAFS data. According to the imaging XAFS analysis, the chemical form of Rh which was located together with Ru was mainly oxide (RhO ). It suggests that stable (Ru,Rh)O
). It suggests that stable (Ru,Rh)O solid solution exists in the simulated glass. On the other hand, that of Rh of which distribution did not accord with Ru in the glass was mainly metallic. In the case of metallic Rh in the glass, it tended to become an aggregation form. It can be concluded that the chemical state of Rh was much affected by the existence and distribution of Ru element.
solid solution exists in the simulated glass. On the other hand, that of Rh of which distribution did not accord with Ru in the glass was mainly metallic. In the case of metallic Rh in the glass, it tended to become an aggregation form. It can be concluded that the chemical state of Rh was much affected by the existence and distribution of Ru element.
Suzuki, Tomoya; Morita, Keisuke; Sasaki, Yuji; Matsumura, Tatsuro
Separation Science and Technology, 51(17), p.2815 - 2822, 2016/09
Times Cited Count:7 Percentile:23.23(Chemistry, Multidisciplinary)To understand the adsorption properties of styrene-divinylbenzene copolymer functionalized with 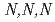 -trimethylglycine, AMP03, the adsorption behaviors for platinoid ions (Ru(III), Rh(III), and Pd(II)) were examined. Furthermore, we performed adsorption experiments using sample solutions with adding triethylamine, thiourea, and
-trimethylglycine, AMP03, the adsorption behaviors for platinoid ions (Ru(III), Rh(III), and Pd(II)) were examined. Furthermore, we performed adsorption experiments using sample solutions with adding triethylamine, thiourea, and 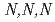 -trimethylglycine. Based on the adsorption data obtained in this study, we performed chromatographic experiments. The results indicated that all platinoid ions in the feed solution completely adsorbed on AMP03, and almost 80% of the adsorbed platinoid ions were recovered. These results show that AMP03 has the potential to recover Ru(III), Rh(III), and Pd(II) from high-level liquid waste.
-trimethylglycine. Based on the adsorption data obtained in this study, we performed chromatographic experiments. The results indicated that all platinoid ions in the feed solution completely adsorbed on AMP03, and almost 80% of the adsorbed platinoid ions were recovered. These results show that AMP03 has the potential to recover Ru(III), Rh(III), and Pd(II) from high-level liquid waste.
Morita, Yasuji; Yamaguchi, Isoo; Fujiwara, Takeshi; Mizoguchi, Kenichi*; Kubota, Masumitsu*
JAERI-Research 2000-024, 55 Pages, 2000/06
no abstracts in English
Morita, Yasuji; Mizoguchi, Kenichi*; Yamaguchi, Isoo; ; Kubota, Masumitsu
JAERI-Research 98-046, 18 Pages, 1998/08
no abstracts in English
; ; *; *; Masaki, Toshio; Kobayashi, Hiroaki; *
PNC TN8410 98-041, 185 Pages, 1998/02
The 9 test of Joule-Heated Cylindrical Electrode Melter - Engineering Scale (JCEM-E9 Test) was carried out from June to July 1996, as a part of the development program on an advanced glass melter. The principal purpose of the test was to estimate the effect of noble metal on operation of the melter with simulated high-level liquid waste. Besides, we also evaluated the basic operational characteristics with corrosion of electrodes, qualities of produced glass etc. JCEM-E is an electric glass melter with an internal electrode and an external electrode in a subsidiary furnace. The internal electrode is a rod inserted in the center of external electrode that is a cylindrical tank. The glass is melted by conducting electric current through the molten glass between the internal and external electrodes. The subsidiary furnace is composed of multi-layer refractories inside a metallic casing and is equipped with the resistance heaters. Melting surface area is 0.35 m
test of Joule-Heated Cylindrical Electrode Melter - Engineering Scale (JCEM-E9 Test) was carried out from June to July 1996, as a part of the development program on an advanced glass melter. The principal purpose of the test was to estimate the effect of noble metal on operation of the melter with simulated high-level liquid waste. Besides, we also evaluated the basic operational characteristics with corrosion of electrodes, qualities of produced glass etc. JCEM-E is an electric glass melter with an internal electrode and an external electrode in a subsidiary furnace. The internal electrode is a rod inserted in the center of external electrode that is a cylindrical tank. The glass is melted by conducting electric current through the molten glass between the internal and external electrodes. The subsidiary furnace is composed of multi-layer refractories inside a metallic casing and is equipped with the resistance heaters. Melting surface area is 0.35 m that i8 approximately half of 0.66 m
that i8 approximately half of 0.66 m of TVF melter. In the test, 13 batches of glass was produced and total weight of produced glass was 3663kg. As a result, The maximum processing rate of JCEM-E with simulated HLLW including noble metals was 4.20
of TVF melter. In the test, 13 batches of glass was produced and total weight of produced glass was 3663kg. As a result, The maximum processing rate of JCEM-E with simulated HLLW including noble metals was 4.20 5.60kg/h, and decreased to less than 80 percent compared with JCEM-E8 Test with non-noble metals HLLW. It was considered that the decrease of the rate arose from concentration of current due to non-uniform distribution of noble metals in molten glass. Judging from the balance of feed and draining, and as a consequence of the observation inside the melter after the test, the draining of noble metals from the nozzle was good. As for the quality of glass produced in the test, properties of concern were comparable with those of standard glass of TVF.
5.60kg/h, and decreased to less than 80 percent compared with JCEM-E8 Test with non-noble metals HLLW. It was considered that the decrease of the rate arose from concentration of current due to non-uniform distribution of noble metals in molten glass. Judging from the balance of feed and draining, and as a consequence of the observation inside the melter after the test, the draining of noble metals from the nozzle was good. As for the quality of glass produced in the test, properties of concern were comparable with those of standard glass of TVF.
Myochin, Munetaka; Iwase, Masanori*
PNC TY8604 96-001, 32 Pages, 1996/03
no abstracts in English
Myochin, Munetaka; Kosugi, Kazumasa; Wada, Yukio; Yamada, Kazuo; Seimiya, Hiroshi; Ishikawa, Hirohisa
PNC TN8410 96-071, 86 Pages, 1996/03
None
Morita, Yasuji; Kubota, Masumitsu
Hoshasei Haikibutsu Kenkyu, 2(1-2), p.75 - 83, 1996/02
no abstracts in English
*; ; ; Kawamura, Kazuhiro; Sasage, Kenichi; ; Ouchi, Jin
PNC TN8100 94-002, 104 Pages, 1994/02
no abstracts in English
Kubota, Masumitsu; Yamaguchi, Isoo; Morita, Yasuji; ; Shirahashi, Koichi; ;
Proc. of the Future Nuclear Systems: Emerging Fuel Cycles and Waste Disposal Options; GLOBAL 93, p.588 - 594, 1993/00
no abstracts in English